February 1, 1999
The first newsletter of the year provides me with an opportunity for reminding all CTS participants that honest reporting is the foundation on which the success of this collaborative study is built. Our intention during the last 16 years has been to speed up the knowledge-gaining process, especially concerning the influence of very complex or rarely occurring events, by combining the experience of many centers. This approach has been remarkably successful. My yearly appeal for honest and accurate reporting is directed primarily to new staff members and new study participants. The research efforts of thousands of workers who directly or indirectly contribute to the CTS study would be jeopardized if information on transplant failures were deliberately withheld. We can be proud that none of the CTS results that were published during the last 16 years had to be withdrawn afterwards because they were incorrect. Please help us to uphold this impressive record by reporting your data accurately and completely. To repeat what has been said many times: the CTS is not a competition for the highest success rate. All data are treated as strictly confidential and the results of individual centers are not released. Anyone who cannot subscribe to the principle of complete and honest reporting should please stop participating in order not to invalidate the sincere research efforts of all other study participants.
_______________
A few centers have fallen so far behind with their reporting that we had to inactivate them at the beginning of the year. Their data will not be included in the CTS analyses until we receive a complete update. Centers that are affected by this will find a special note on the current data sendout. For all others, this is an excellent time to check whether the "Data Status" section of the printout shows that all consecutive transplants performed during 1998 are contained in the CTS file. If not, please report any missing cases as soon as possible.
_______________
An increasing number of centers are providing their data in the form of data downloads from local or national databanks. Our computer staff will be happy to assist you if you wish to switch to the electronic data transfer method. In addition to accepting data on CTS report forms, we also accept photocopies of reports to other registries or even printouts of other registry data.
_______________
With more than 200,000 transplants documented in the CTS, a sincere word of thanks is due to all who have contributed. It is not only the size of the study but more importantly the excellent data quality that explains why this collaborative project has become a widely recognized international reference point. Each participating center and each individual contributor is an important part of our collective success. As the numbers of transplants grow, maintaining the data files becomes more difficult, not the least because cost containment measures in the health sector also affect the documentation of organ transplants. It is important to be reminded, however, that without accurate documentation and analysis there will be no further progress, and that this applies both to local research efforts as well as to our large collaborative study.
_______________
I would like to direct special thanks to all those who have provided data on the yearly "Immunosuppression Questionnaires". These data have evolved to become the most productive part of the CTS file. Of course, it is recognized that the extra time and effort required for completing the forms is not trivial. Because the data are so informative, I urge you to continue this important work and I would like to invite those who have not contributed in the past to do so in the future.
An example of the data's usefulness was provided in Newsletter 1/1998 which showed the results of an analysis of posttransplant hypertension. Another example was the data on steroid-free maintenance immunosuppression shown in Newsletter 3/1994. As you may recall, because of questions raised at that time whether retrospective data could be a guideline for prospective intervention, a prospective steroid withdrawal study was initiated within the CTS framework. Today, we are able to present to you a first preliminary 3-year follow up analysis.
Although we planned to have a randomized and a non-randomized study arm, most participants chose the non-randomized arm for reasons of simplicity. For the present analysis, we have therefore combined the randomized and non-randomized patients. The patients were separated into two groups: one in which study participation was clearly prospective, that is, the study registration forms were sent to Heidelberg at the time of commencing steroid tapering. The second group consisted of patients in whom steroid-tapering was initiated some time earlier but who otherwise qualified for the study according to the guidelines laid out in the "Consensus Protocol". The two groups were analyzed separately in order to exclude any potential bias that might be introduced by including "retrospective cases". As it turned out, the results of the two groups were very similar.
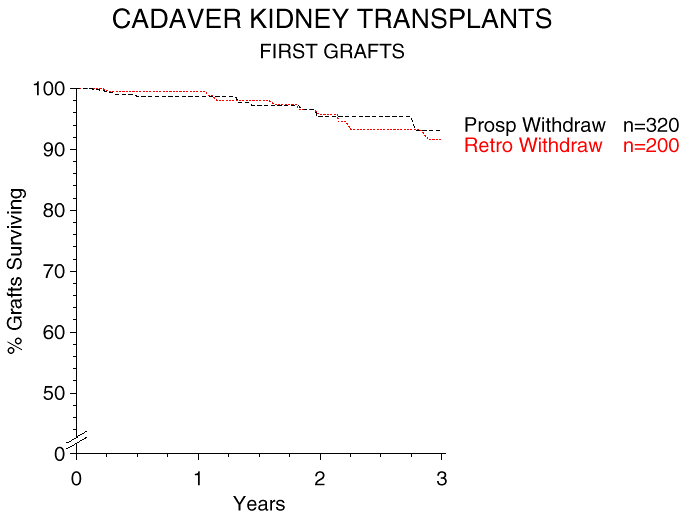
Figure 1 shows a 3-year graft survival analysis of cadaver kidney transplants starting from the day of steroid tapering. The graft survival rate 3 years later was approximately 95%. One year after commmencing steroid tapering, 75% of the patients were on steroid-free maintenance immunosuppression. The 3-year graft survival rate after steroid withdrawal in an additional 96 recipients of one-haplotype matched related donor grafts was 96%.

Figure 1
A parallel analysis of heart transplant recipients is shown in Figure 2. The fraction of patients who were steroid-free one year after entering the program was much lower than in kidney transplant recipients: only 41% of heart recipients were on steroid-free maintenance immunosuppression. It is not clear whether there is a genuine difference between kidney and heart transplants, or whether the lower rate of steroid-free heart patients is the result of a more cautious approach to steroid withdrawal by heart transplant physicians.
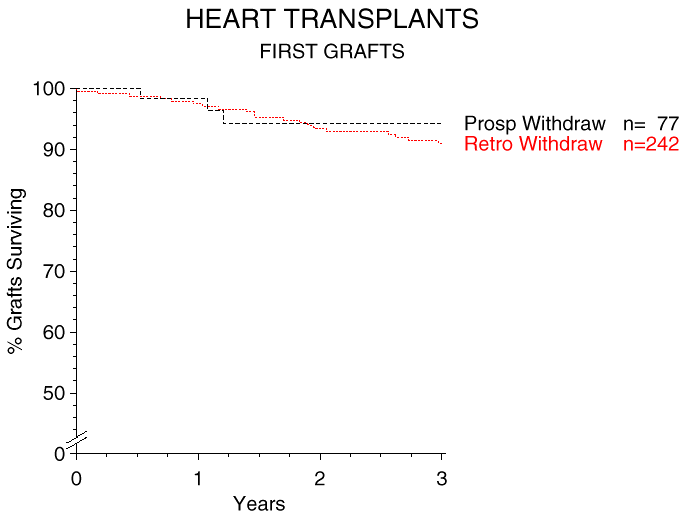
Figure 2
_______________
Altogether, these preliminary 3-year data show that the results of the prospective study closely match those of the previously published retrospective CTS data on steroid-free maintenance. Patients who can be taken off steroids have an excellent outcome. From the data gathered so far, it appears that approximately 75% of kidney recipients and 40% of heart recipients can be maintained on a steroid-free longterm regimen. Importantly, the "intention to treat" analysis does not show evidence for a detrimental effect on the overall graft survival rate, thus indicating that there was no excessive graft loss in patients who had to be returned to steroid treatment because of rejection.
There remains a questionmark concerning longterm outcome because of the results of a previous Canadian multicenter study which showed that kidney graft function deteriorated in steroid-free patients during the fifth year after steroid withdrawal. It will therefore be very important to obtain complete follow up data during the years to come. Because the retrospective CTS data on steroid-free patients do not show any negative influence on graft outcome even after 7 years of follow up, we believe that the risk of such deterioration occurring in the prospective collaborative study must be small. Nevertheless, complete documentation for 5 years and longer is an important goal of this project.
We are continuing to accept new patients for the steroid withdrawal study. With the 3-year results now at hand, it is clear that steroid withdrawal under controlled conditions is not associated with an excessive risk of graft loss. I am therefore hoping that many more centers will decide to prospectively enroll patients who fulfill the criteria of eligibility. In addition to strengthening the information concerning graft survival, we will need to perform detailed analyses on the influence of steroid-free treatment on side effects, such as the incidence and severity of osteoporosis, cataracts, cholesterol status, hypertension, and any changes in the rate and cause of death after longterm immunosuppressive treatment. By enrolling your patients you will not only help us generate additional important data, but you will in all likelihood do something beneficial for those patients who can be taken off steroids.
_______________
We would like to thank the laboratories that contributed more than 1,800 donor and recipient samples during the last cycle of the DNA project. Please continue to support this project. In addition to samples from kidney transplants, we would like to investigate as many heart and liver transplants as possible. The next shipping date is:
May 03/04, 1999.
It is never too late for collecting material. There will be another shipping cycle in the fall. If you need instructions, please do not hesitate to contact us.
_______________
Information on parental HLA typings for the "sibling project" continues to arrive. If you have not yet returned the questionnaire, please do so soon. For this special project, information on even a single transplant is valuable. We will compile and analyze the results in time for inclusion in the next CTS newsletter.
_______________
Thank you for your cooperation.
With all best wishes for a successful year 1999,
Sincerely yours,