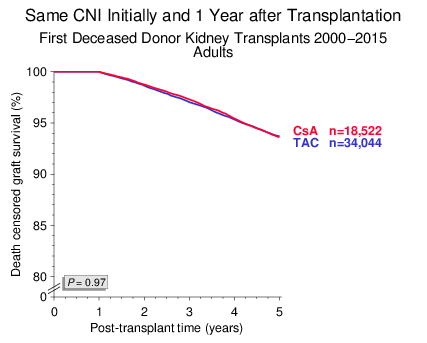
Figure 1
CTS Collaborative Transplant Study
Dear Colleague
The vast majority of kidney transplant recipients are currently receiving tacrolimus (TAC)-based
maintenance immunosuppression. Graft survival analyses based on CTS data, however, repeatedly
failed to demonstrate superiority of TAC-based regimens over cyclosporin A (CsA)-based
immunosuppression (CTS Newsletter 2:2009; Opelz G and Döhler B. Influence of immunosuppressive
regimens on graft survival and secondary outcomes after kidney transplantation.
Transplantation 87: 795–802, 2009). We dedicated this newsletter to a reanalysis of the impact
of maintenance immunosuppression from year 1 to 5 with these two calcineurin inhibitors (CNIs).
Recipients of deceased donor kidneys who were transplanted during 2000–2015 and were treated initially and at year 1 post-transplantation with CsA or TAC were analyzed. Patients who participated in a blinded trial, recipients of combined organ transplantations and patients receiving mTOR inhibitor medication during the first post-transplant year were excluded. Death censored graft survival from year 1 to year 5 was analyzed using univariate Kaplan-Meier and multivariable Cox regression methodologies. For the latter, the following confounders were considered: geographical region, transplant year, transplant number, recipient and donor age, sex and race, pretransplant time on dialysis, HLA mismatches, cause of donor death, donor history of hypertension, pretransplant antibodies, cold ischemia time, antibody induction therapy, rejection treatment during year 1. Additionally, serum creatinine, blood pressure, serum cholesterol and treatment for diabetes at year 1 were included as confounders.
68,172 transplantations fulfilled the inclusion criteria. Initially and at year 1, 30.6% of the patients were on a CsA-based and twice as many (62.1%) on a TAC-based regimen. During the first year, 5.8% of the patients were switched from CsA to TAC and only 1.5% from TAC to CsA.
We first analyzed adult recipients of first kidney transplants in whom the type of CNI was not changed during the first post-transplant year. As shown in Figure 1, death censored graft survival of CsA- and TAC-treated patients from year 1 to 5 was virtually identical. This result was confirmed in multivariable Cox-regression analysis: hazard ratio (HR) of TAC vs. CsA 0.99, with a 95% confidence interval (CI) of 0.91–1.07, P=0.73.

Figure 1
Patient survival was also not different in patients who received a TAC- or CsA-based regimen (Figure 2).
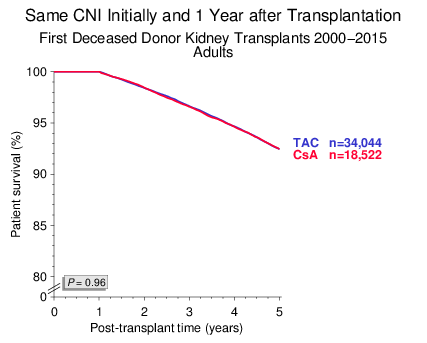
Figure 2
The fraction of patients with a creatinine value of <130 µmol/L at year 1 was 3 percentage points higher among TAC-treated patients (55.3% vs. 52.4%, P<0.001). When patients were stratified for 1-year creatinine of <130 or ≥130 µmol/L, subsequent death censored graft survival did not differ significantly between TAC- or CsA-treated recipients (Figure 3): (a) HR 0.92, 95% CI 0.78–1.09, P=0.35, (b) HR 1.00, 95% CI 0.92–1.10, P=0.92.
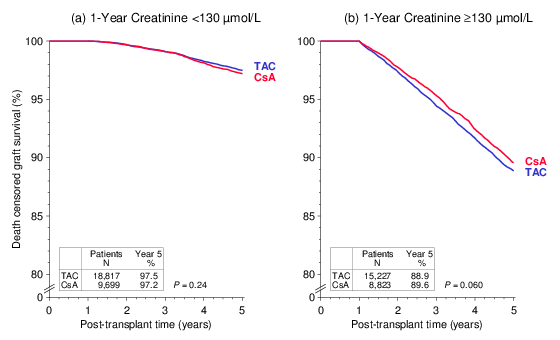
Figure 3
Currently, apparently because TAC is considered more effective, CsA is rarely used in children and retransplant recipients who are considered to be at increased risk of graft loss due to higher immune reactivity. The numbers of patients treated with the respective CNI shown in the figures below are rather convincing. Surprisingly, when these two patient groups at increased immunological risk were analyzed, death censored graft survival from year 1 to 5 was significantly better in patients who received a CsA-based regimen initially and at year 1 than in patients who were on TAC-based immunosuppression (Figure 4).
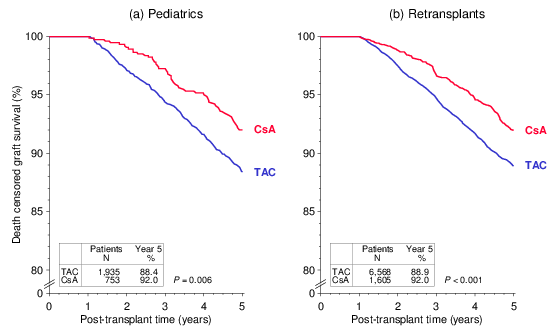
Figure 4
These findings were confirmed in multivariable Cox regression analyses: (a) HR 1.44, 95% CI 1.04–1.97, P=0.026, (b) HR 1.41, 95% CI 1.15–1.72, P<0.001. The hazard ratios indicate a more than 40% higher risk of graft loss in patients who were on a TAC-based regimen. As shown in Figure 5, the superiority of CsA over TAC in these two patient groups is consistent in subgroup analysis of recipients on triple immunosuppression including mycophenolic acid (MPA) and steroids (STE). The results are also consistent in patients with good kidney function (creatinine of <130 µmol/L) at year 1 and no rejection treatment during the first year and in patients with impaired kidney function (creatinine of ≥130 µmol/L) at year or rejection treatment during the first year. Whether treatment with insufficient doses of CNI, MPA or STE might explain the inferior outcome in patients at increased risk who received TAC-based immunosuppression needs to be addressed in further analyses.
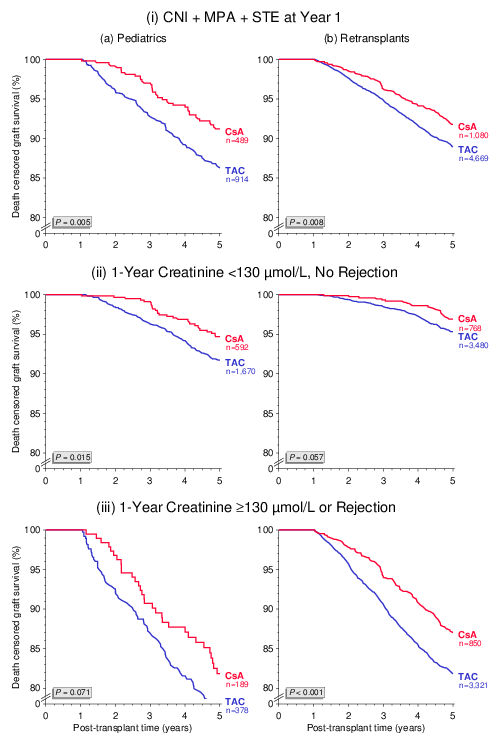
Figure 5
A common argument for superiority of TAC in preventing graft loss is that TAC is more often used in patients at increased risk of rejection and that patients on CsA are more frequently switched to TAC than the other way around. We also found in the current analysis that more patients were switched from CsA to TAC during the first post-transplant year than from TAC to CsA. However, death censored graft survival was virtually identical in both switch groups: HR 0.95, 95% CI 0.75–1.20, P=0.66 (Figure 6).
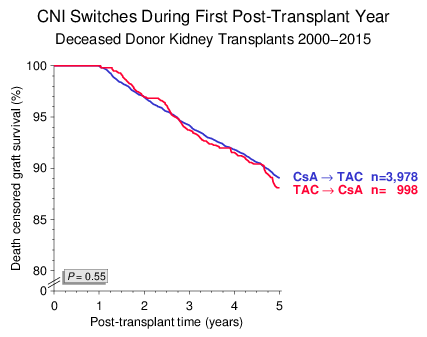
Figure 6
In these analyses, the reason why one or the other of the two CNIs was chosen is not available and this could be considered a limitation. Nevertheless, we did not observe superiority of TAC over CsA in terms of death censored graft survival from year 1 to 5 in any of the analyzed comparisons.
If you are not currently participating in the CTS DNA and Serum Studies, please consider doing so in the future. These studies are revealing results with important implications for the clinical treatment of transplant recipients. The next shipping date for DNA and serum is
June 18/19, 2018.
Thank you very much for your support of the CTS!
Sincerely yours,

Caner Süsal